A new somatostatin receptor antagonist in the diagnostic algorithm of neuroendocrine tumors – feasibility study
 Project title
Project title
A new somatostatin receptor antagonist in the diagnostic algorithm of neuroendocrine tumors – feasibility study
 Name of Beneficiary/Beneficiaries
Name of Beneficiary/Beneficiaries
National Center for Nuclear Research
TECANT project partners implement contracts financed by authorized national institutions, including: Austrian Science Fund FWF (project I 4220-B) for the Medical University in Innsbruck; the NCBR under contracts ERAPerMed/01/2019 for Jagiellonian University / Collegium Medicum and ERAPerMed/02/2019 for the National Nuclear Research Center; MIZS (Ministry of Education, Science and Sport) project C3330-19-522011 for the University of Ljubliana and the University Medical Centre of Ljubljana.
 Name of programme
Name of programme
International programs
 Competition
Competition
ERA PerMed JTC 2018
 Project value
Project value
PLN 523,787.50
 Funding value
Funding value
PLN 523,787.50
 Project delivery period
Project delivery period
01.07.2019 – 30.06.2023
Our team
Partners of the international TECANT project during the meeting in Barcelona in 2019
Results of our work
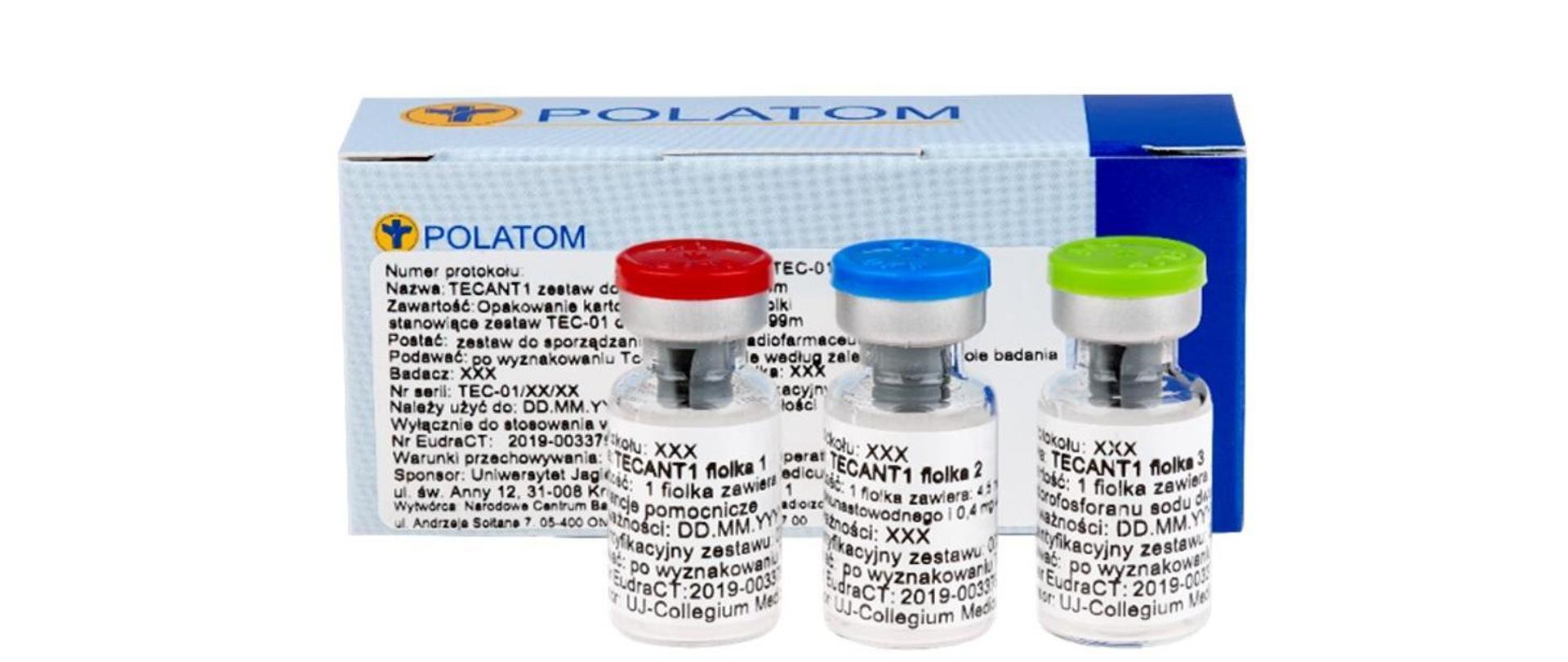
The result of the work was the development of a kit of 3 vials containing lyophilisate that facilitates material marking with technetium-99m in clinical conditions, allowing to obtain a stable product with high radiochemical purity, ready for administration to patients.
TECANT-1 3-vial kit for material marking with technetium-99m
For the first time, the results of SPECT/CT imaging after administration of the investigated radiopharmaceutical 99mTc[Tc]-TECANT-1 in a patient with neuroendocrine tumor were shown in the publication: Opalinska, M., Lezaic, L., Decristoforo, C. et al.: “Comparison of 99mTc radiolabeled somatostatin antagonist with [68 Ga]Ga-DOTA-TATE in a patient with advanced neuroendocrine tumor”, Eur J Nucl Med Mol Imaging 50, 4110–4111 (2023), https://doi.org/10.1007/s00259-023-06335-9.
Comparison of SPECT/CT imaging using a new radiopharmaceutical, the SSTR antagonist, [99mTc]Tc-TECANT1 (top panel), with PET/CT imaging using the agonist [68Ga]Ga-DOTA-TATE (bottom panel). It should be noted that there was a more pronounced accumulation of [99mTc]Tc-TECANT1 in lesions (6 out of 7 lesions were visualized) as well as a significantly higher ratio of accumulation in the lesions in relation to the background compared to the PET/CT examination with [68Ga]Ga-DOTA-TATE.
Issues addressed
The aim of the TECANT project was to develop a modern radiopharmaceutical – a 99mTc-labeled SSTR antagonist – as a new tool for imaging diagnostics of NETs, which would enable optimization of localization diagnostics and individualization of therapeutic treatment in patients with this cancer.
In the first stage of the project, preclinical tests were carried out on two SSTR antagonists – LM-3: p-Cl-Phe-cyclo(D-Cys-Tyr-D-Aph(Cbm)-Lys-Thr-Cys)D-Tyr-NH2 (TECANT-1) and p-Cl-BASS: p-Cl-Phe-cyclo(D-Cys-Tyr-D-Trp-Lys-Thr-Cys)D-Tyr-NH2 (TECANT-2), oba połączone z chelatorem N4. [99mTc]Tc-TECANT-1 showed more favorable imaging properties compared to [99mTc]Tc-TECANT-2 and, therefore, for this peptide, research was undertaken to develop a pharmaceutical kit for the preparation of a radiopharmaceutical labeled with technetium-99m. The effect of the project was: preparation of a pharmaceutical in the form of a 3-vial set for use in clinical conditions (in the form of a kit for obtaining a radiopharmaceutical); development of an optimal technetium-99m labeling procedure; collecting data necessary to develop a dossier containing product safety data obtained in the preclinical part of the study (Investigational Medicinal Product Dossier, IMPD). Based on the submitted documentation, a permission was obtained from the Office for Registration of Medicinal Products, Medicinal Products and Biocidal Products (N° UR/DBL/D/260/2022, date on November 10, 2022) to conduct the TECANT clinical trial titled: “New antagonists of somatostatin receptors labeled with Tc-99m in the diagnostic algorithm of neuroendocrine tumors – a detection study”. Kits of the investigational medicinal product prepared at the National Center for Nuclear Research were delivered to centers in Kraków, Innsbruck and Ljubljana responsible for conducting the clinical trial. In the first phase clinical trial, EudraCT number (2019-003379-2), ten patients with NETs and confirmed SSTR expression based on imaging with a labeled agonist underwent SPECT/CT imaging after administration of [99mTc]Tc-TECANT-1. The project assessed the safety of administration of the tested biomarker, its pharmacodynamics and pharmacokinetics, and performed a dosimetric analysis along with the development of a repeatable method for the quantitative analysis of the obtained data.
Project beneficiaries
Localization diagnosis of neuroendocrine tumors (NETs) and monitoring response to treatment is a challenge for clinicians due to the significant cellular heterogeneity of these tumors and unpredictable clinical course. Preliminary preclinical studies suggested that new radiopharmaceuticals based on somatostatin receptor (SSTR) antagonists may provide better visualization of NETs and assessment of the level of SSTR expression compared to widely used SSTR agonists. The introduction of SSTR antagonists into clinical practice would constitute a significant progress in the treatment of patients with this cancer. The results of the phase 1 clinical trial carried out as part of the TECANT project confirmed the high diagnostic value of SPECT/CT examination after administration of the investigated radiopharmaceutical [99mTc]Tc-TECANT-1 in patients with neuroendocrine tumors.
Major implementation challenges
The project began during the period of restrictions related to the Covid-19 pandemic. Difficulties in communication with the supplier of the TECANT-1 peptide and in access to laboratories due to the lockdown resulted in a delay in the work.
In order to have [99mTc]Tc-TECANT-1 admitted to clinical trials, it was necessary to submit a protocol from an extended toxicological study covering the fields of hematology, clinical biochemistry and histopathology. For this reason, a GLP-certified specialized institution was involved in the implementation of the task on a contract basis. Single dose toxicity studies of TECANT-1 were conducted in accordance with ICH EMA guideline M3 R2 (CPMP/ICH/286/95). Such research takes a long time to complete.
Our advice to other applicants
Projects aimed at confirming the effects of scientific research / preclinical research in a clinical trial require meeting a number of formal requirements and obtaining permits from authorized offices, which may take longer than the officially declared official procedure. Applicants usually allow a certain amount of time to obtain these documents, but this margin must not be too wide and the stages of the administrative procedure must not go beyond the project time frame. When applying for projects, the risk of not obtaining appropriate permits within the assumed deadline should be taken into account.


