Advanced Biomaterial and Biofabrication Method for engineering the myotendinous junction
 Project title
Project title
Advanced Biomaterial and Biofabrication Method for engineering the myotendinous junction
 Name of Beneficiary/Beneficiaries
Name of Beneficiary/Beneficiaries
Warsaw University of Technology
 Name of programme
Name of programme
Internetional programs
 Competition
Competition
6th Polish Taiwanese / Taiwanese-Polish Joint Research Call
 Project value
Project value
PLN 385 607,98
 Funding value
Funding value
PLN 385 607,98
 Project delivery period
Project delivery period
01.02.2019 – 31.05.2023
Results of our work
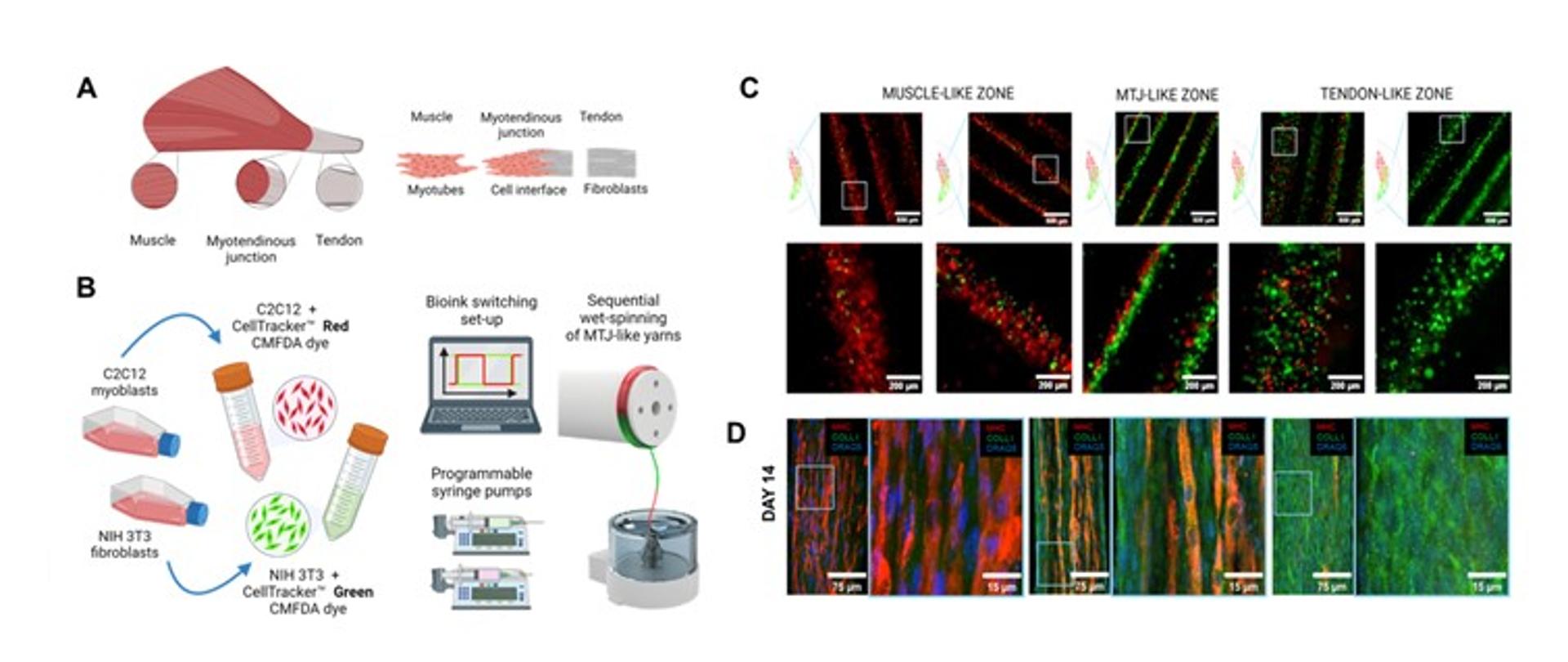
Figure 1. Engineering the MTJ using sequential microfluidic wet spinning. A) Schematics of the native anatomy of the muscle-tendon interface. B) Labelling process of C2C12 myoblasts and NIH 3T3 fibroblasts with viable red and green cell tracker, respectively; seequential wet-spinning process of cell-laden hydrogel microfibers. C) Fluorescence images of C2C12 myoblasts and NIH 3T3 fibroblasts and their distribution within the microfiber core. D) Representative confocal images of myotendinous junction (MTJ)-like C2C12 myoblasts/NIH 3T3 fibroblasts yarns stained for myosin heavy chain (MHC) (red), collagen type I (COLLI) (green), collagen type III (COLL III) (green), and DRAQ5 (blue) at day 14 of cell culture.
In this work, we developed MTJ-like hydrogel yarns via a novel microfluidics-assisted 3D printing wet-spinning technique combined with programmable syringe pumps to rapidly switch m-ink and t-ink. By fine-tuning the wet-spinning parameters, we obtained biomimetic, highly compartmentalized scaffolds that effectively promoted anisotropic alignment of both muscle and tendon precursor cells. The sequential wet-spinning of C2C12 myoblasts and NIH 3T3 fibroblasts provided a controlled gradient pattern that effectively replicated the biological composition and arrangement of the muscle-tendon interface. Localized expression of tissue-specific markers identified muscle and tenogenic maturation in the designed scaffold regions. Furthermore, interdigitation of mature muscle myofibers within the tenogenic extracellular matrix was detected at the level of the transition zone. Such findings validate our biofabrication method as a potential engineering platform for the regeneration of the muscle-tendon interface in vitro.
Issues addressed
Our project addresses the challenge of effectively treating and regenerating the muscle-tendon junction (MTJ), a critical biomechanical interface that is highly prone to injury due to its unique architecture and biomechanical demands. Injuries at the MTJ, such as tears or ruptures, often result from overloading or repetitive strain, especially in athletes and aging populations, leading to compromised mobility and reduced quality of life. The current clinical treatments, including surgical suture repairs and grafting (autografts/allografts), while effective in reattachment, often result in fibrotic scar formation, incomplete recovery, or donor site morbidity, and can increase the risk of reinjury. To overcome these limitations, our project introduces an innovative biofabrication strategy to replicate the native MTJ's structural complexity and biomechanical properties in vitro. Using a novel automated microfluidics-assisted wet-spinning method, we developed a bi-compartmentalized scaffold designed to mimic the muscle-tendon interface. This scaffold incorporates aligned core-shell hydrogel microfibers that replicate the smooth transition between muscle, tendon, and the MTJ, promoting proper cellular orientation, alignment, and tissue differentiation. By optimizing the wet-spinning parameters and using advanced microfluidic and 3D printing technologies, we have produced a platform that can guide the development of engineered muscle and tendon tissues, providing a more functional solution for MTJ repair. Our project addresses the limitations of current clinical treatments by offering a scaffold that facilitates better tissue integration, reduces fibrosis, and mimics the native MTJ, thereby potentially improving long-term recovery and reducing reinjury risks. This biofabrication platform represents a promising alternative to conventional methods and aims to provide a more effective, biocompatible solution for MTJ injuries.
Project beneficiaries
The primary beneficiaries of this research are tissue engineers and the broader scientific community, as it provides a novel biofabrication platform for studying and replicating the complex muscle-tendon junction (MTJ). This platform advances current in vitro models by enabling more accurate tissue compartmentalization and anisotropic cell alignment, offering new avenues for investigating MTJ biology, injury mechanisms, and potential therapies.
While clinical applications are still distant, this research holds the prospective potential to benefit patients with muscle-tendon injuries in the future by paving the way for more effective and functional treatment options. In the short term, it serves as a valuable tool for researchers to explore tissue regeneration strategies and improve our understanding of MTJ repair and regeneration.
