Interdisciplinary Environmental Doctoral Studies in “Physical, Chemical and Biophysical Foundations of Modern Technologies and Materials Engineering” (FCB)
 Project title
Project title
Interdisciplinary Environmental Doctoral Studies in “Physical, Chemical and Biophysical Foundations of Modern Technologies and Materials Engineering” (FCB)
 Name of Beneficiary/Beneficiaries
Name of Beneficiary/Beneficiaries
Institute of Catalysis and Surface Chemistry of the Polish Academy of Sciences in Krakow
 Name of programme
Name of programme
“Knowledge, Education, Development” Operational Program
 Competition
Competition
Interdisciplinary doctoral studies
 Project value
Project value
PLN 10,123,883.00 for 75 people
 Funding value
Funding value
PLN 134,895.00 per person
 Project delivery period
Project delivery period
01/09/2017 – 31/08/2022 (extended until 31/10/2023)
Meet our team
- M.Sc. Agnieszka Winiarska (postgr. student of FCB studies at IKiFP PAN)
- Prof. Sc.D. Maciej Szaleniec (IKiFP)
- Prof. Sc.D. Anna Bodzoń-Kułakowska (AGH)
- Sc.D. Joanna Kryściak-Czerwenka (IKiFP)
See the result of our work
The results of research on oxidoreductase of aldehyde (AOR) from the bacterium Aromatoleum aromaticum:
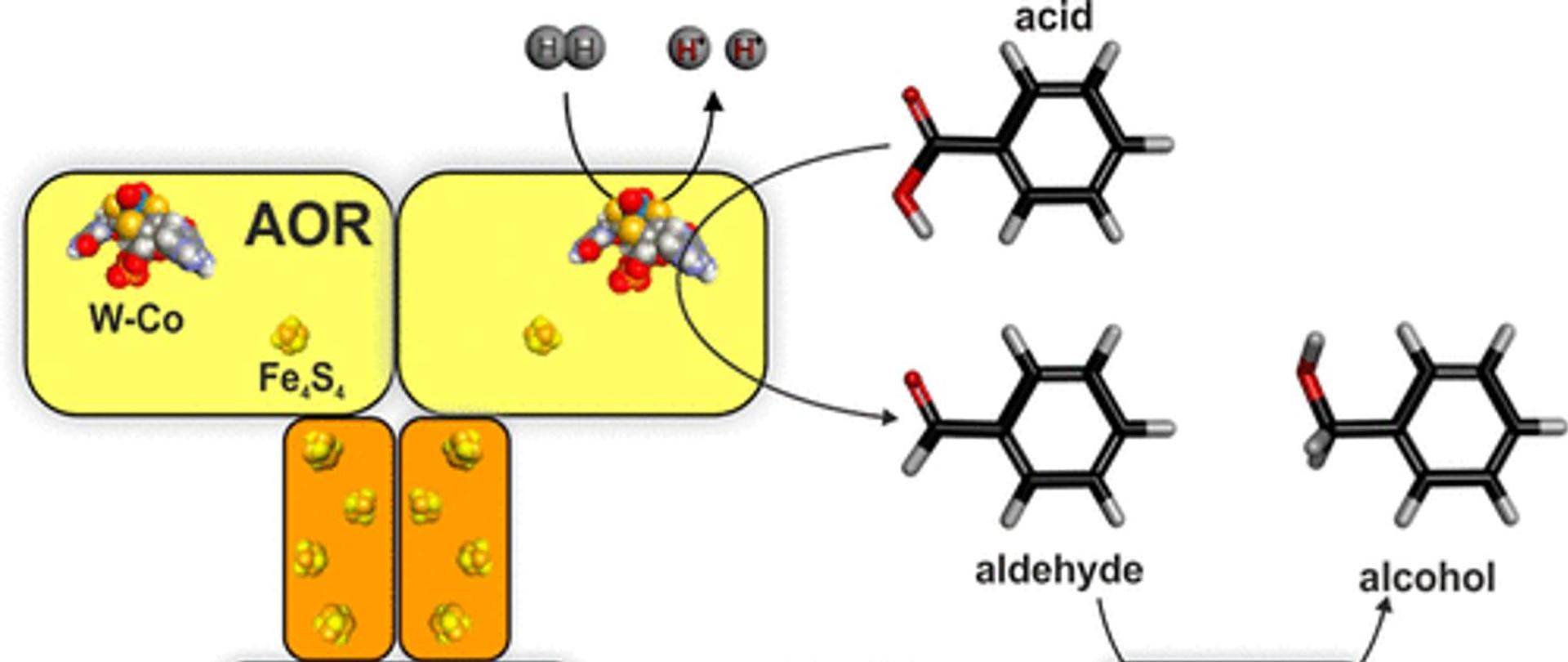
- A new biocatalyst for the selective reduction of carboxylic acids to aldehydes using low concentrations of hydrogen as the sole electron donor. The aldehydes produced include vanillin, benzaldehyde, nicotinaldehyde, short aliphatic aldehydes.
- New biocatalytic system for the regeneration of the reduced form of the nicotinamide adenine dinucleotide cofactor used to reduce compounds by means of oxidoreductases.
- Construction of an in vitro enzyme cascade for the production of alcohols from carboxylic acids using hydrogen as the sole electron donor;
- Solution of the AOR structure by cryo-electron microscopy and determination of part of the structure-function relationship of the complex.
Project results were published in the ACS Catalysis journal, vol. 13, 2022:
Tungsten Enzyme Using Hydrogen as an Electron Donor to Reduce Carboxylic Acids and NAD+
Agnieszka Winiarska, Dominik Hege, Yvonne Gemmecker, Joanna Kryściak-Czerwenka, Andreas Seubert, Johann Heider, Maciej Szaleniec
The results include the Polish and European patent applications:
- A. Winiarska, J. Heider, M. Szaleniec. D. Hege, F. Arndt „Sposób enzymatycznej redukcji formy utlenionej dinukleotydu nikotynowo-adeninowego.” Polish Patent Application P.437449 (29.03.2021)
- A. Winiarska, J. Heider, M. Szaleniec. D. Hege, F. Arndt „Sposób otrzymywania aldehydów poprzez enzymatyczną redukcję kwasów karboksylowych”, Polish Patent Application P.437445 (29.03.2021)
- A. Winiarska, J. Heider, M. Szaleniec. D. Hege, F. Arndt, A.Wojtkiewicz, „A Method of Enzymatic Reduction of the Oxidized Nicotinamide Adenine Dinucleotide and Carboxylic Acids”, 2022; Vol. 4, EP22164459
What problem does our project solve?
The use of enzymes as biocatalysts in production processes allows to reduce the applied temperatures and pressures and increase the selectivity of the synthesis method in relation to chemical methods. As a result, processes can be cheaper (less energy-intensive) and their products can have a smaller carbon footprint and higher purity. The main objective of the project was to use oxidoreductases derived from the bacterium Aromatoleum aromaticum for the production of bioalcohols from carboxylic acids. The enzymes were tungsten aldehyde oxidoreductase (AOR) and benzyl alcohol dehydrogenase (BaDH). The activities of both enzymes were studied in detail, resulting in the discovery of new AOR activity as hydrogenase. The AOR enzyme in the presence of hydrogen was found to catalyze the reduction of carboxylic acids to aldehydes or the reduction of oxidized nicotinamide adenine dinucleotide (NAD+) to its reduced form (NADH).
The developed method allows for selective production of alcohols using only hydrogen as a pure and renewable reducer for the entire process, in which the catalysts were aldehyde oxidoreductase and alcohol dehydrogenase derived from the bacterium Aromatoleum aromaticum.

The reduction of carboxylic acids catalyzed by AOR may also be a stage for obtaining other valuable chemical compounds for which aldehyde is an intermediate product in synthesis. For example, converting alcohol dehydrogenase to aldolase would allow the production of dihydroxy-α-ketoacids.
Tungsten enzymes, such as AORs, are a relatively unknown group of proteins. For some of them, their activity has not yet been determined, and only a few structures of such proteins have been solved so far, and with low resolution for the most important fragment, i.e. the active center containing the bound tungsten ion. Our project particularly contributed to the understanding of the catalytic capabilities and structure of the AOR.
Who benefits / will benefit from the results of the project?
New AOR activities can be used in the synthesis of bioalcohols, aldehydes and their derivatives used in the food and pharmaceutical industries and in modern (high-tech) materials. Due to the selectivity of the acid reduction process and the pure method of NADH regeneration, AOR has great potential for use as a biocatalyst in new pathways of synthesis of drugs, food additives (vanillin) and other valuable compounds.
What was the biggest challenge for us in the project?
The new activity of AOR as hydrogenase in carboxylic acid reduction discovered by us is a reaction that is seemingly thermodynamically unlikely to occur due to the large difference in acid reduction potential and hydrogen oxidation. Therefore, proving the new catalytic activity of AOR required an additional number of experiments that helped explain why this reaction occurs even at low hydrogen concentrations.
In addition, due to the sensitivity of the biocatalyst (AOR) to atmospheric oxygen, the characterization of the catalyst and the development of a aldehyde synthesis method required the use of anaerobic conditions. Both biocatalyst purification and synthesis were carried out in an anaerobic chamber and using a special working methodology to avoid contact with oxygen.
Determination of the operating conditions of the reactor (hydrogen concentration, substrate, pH) for the production of bioalcohols required precise characterization of the reactions catalyzed by both enzymes.
Studies of the AOR structure were challenging both because of the enzyme’s sensitivity to oxygen and because of the lack of protein crystallization (which made it impossible to solve the structure by X-ray diffraction). To solve this problem, we conducted structure studies using cryo-electron microscopy and mass photometry in collaboration with the Synmikro Center in Marburg, Germany. At the same time, we solved the structure of the active center by reinterpreting the 25-years-old electron density map from AOR from P. furiosus using QM:MM modeling. In this way, we could introduce the tungsten cofactor structure into our model.